What is the function of surfactants?
What is the function of surfactants?
Surfactants are active cleansing agents that play three main roles:
- Penetrate the fabric and wet the fabric
- Loosen and remove dirt and grime (this feature helps the mechanical activity of the washing machine)
- Emulsifying dirt and dirt particles and keeping them suspended in the washing environment
How do surfactants work?
There are two domains in each surfactant molecule: a polar or hydrophilic part and a non-polar or fatty or hydrophobic part. A basic and important principle is that polar materials interact well with other polar materials, and the same is true for non-polar materials.
- Penetrate and wet the fabric
Water has a high surface tension, which prevents many waves from forming on its surface (air-water, water-oil, water-solid). As soon as the surfactant breaks the bond between the water molecules, the surface tension of the water decreases and the water can become distorted, leaving more surfactant molecules near the surface of the water. Now water can seep on surfaces that have not been wet before, such as fabrics.
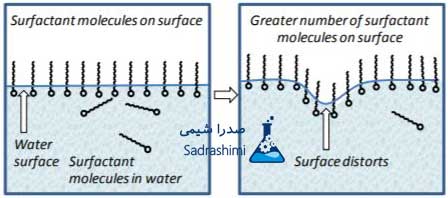
Figure 1: Surfactants reduce the surface tension of water.
- Separation and emulsification of pus and contamination
Normally water and oil do not mix with each other. Oil is a non-polar substance while water is polar. However, in the presence of sufficient amounts and concentrations of surfactant molecules, the oil can be effectively emulsified in water.
Therefore, the polar part of the surfactant molecule is absorbed by water and the non-polar part is absorbed by fat. Surfactant molecules accumulate around the fat and when they are large enough, they form a spherical micelle. The fat particles in the micelles stay in the water well. The process is repeated until sufficient amounts of surfactant molecules are available to interact with the fat residues.